Expandable IV
Medical Device Design/ Research/ Modeling, InVenture Prize Golden Ticket Project (2020) , Sponsored by Georgia Clinical & Translational Science Alliance
In high pressure situations, health-care providers should be able to insert large diameter IVs without hassle to maximize fluid delivery in short amounts of time. Thus, there is a need to design an IV catheter system that is more reliable, safer, and easier to operate while still being able to deliver fluids at necessary rates.
We designed an expandable IV that provides nurses and physicians the option of using smaller sized needles for easier venous access while still being able to deliver large quantities of fluid.
(2021)
Three Design Phases
Translating User Needs into Design Inputs
We interviewed 30+ health professionals nurses and physicians, and translated their needs into quantifiable user inputs with established metrics. We considered these design inputs heavily during our ideation and prototyping phase to ensure that our device meets the necessary requirements to function and satisfy user needs.
Ideation and Prior Art
Apart from generating concept sketches, we examined the relevance of prior art on expandable intravenous catheters to our team’s unique catheter design concepts. In addition, we briefly explore the metrics used to objectively decide on the top design concept.
We have designed a double-layered cannula with bare metal stent mesh that could increase flow rate threefold as compared to a traditional cannula.
Solidworks Animation
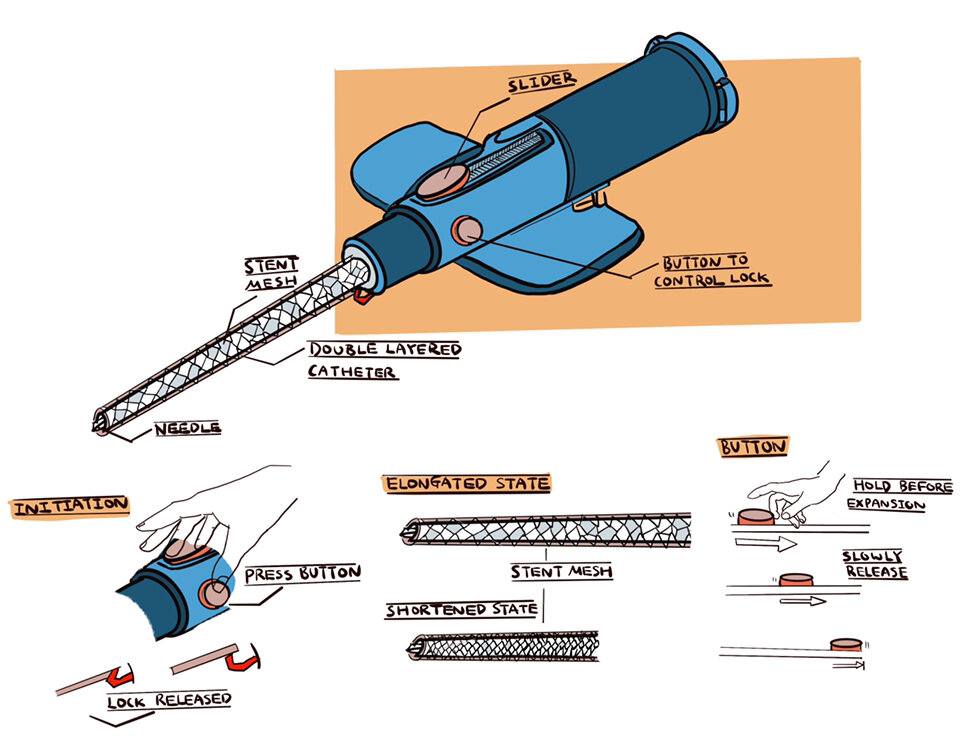
The design implements the novel use of a stent-mesh between a double layered cannula to support the tube’s structure during expansion. The stent was added to the design model along with a button activated lock to keep the structure rigid. This was to maintain the elongated structure of the cannula while providing users the option to disengage the lock and using the slider on the device to transition the device into the expanded state which enables the same flow rate of 180mL/min as an industry standard 16 gauge catheter.
Detailed Expansion Mechanism
Pre-Expansion
The catheter in its initial state matches the parameter of an industry standard 20 gauge catheter. In this state, the elastic silicone cannula tubing is elongated to attain a length of 32mm and external diameter of 1.1mm. This configuration is then bounded by a lock on the catheter hub to prevent the tube from snapping back.
Expansion
The lock can be disengaged by pushing in both side buttons to expand the cannula. Once disengaged, the stent mesh is compressed by the elastic force of the cannula causing the cannula tubing to shorten and consequently expand radially. This expansion process is controlled by the user through a slider mechanism allowing for a gradual change in cannula size rather than an instantaneous snap back of the elastic tube.
Post-Expansion
The post-expansion catheter in its shortened state has a length of 18.9mm and an external diameter of 1.27mm. This post-expansion model would result in a threefold increase in flow rate from 60 ml/min to 180 ml/min.
Concept Development

initial concepts (#1-6)

initial concepts (#7-15)

Top 3 concepts (#1)

Top 3 concepts (#2)

Top 3 concepts (#3)
Final Design

Solidworks CAD

Keyshot Render
Testing
Predicate Testing
We tested flow rates of pre and post expansion on a 4x cannula model. We ran 32 trials in total and recorded time taken for 200ml water in the IV bags to pass through the cannula.
Fluid Simulation
We ran fluid simulation in Solidworks to cross examine flow rate in 1x original catheter model. The results verified that the proposed design at its two different states is indeed capable of providing the same flow rate as industry standard 16 gauge and 20 gauge catheters with an increase in fluid flow velocity.
Original Cannula at expanded state with larger diameter.
Elongated Cannula with smaller diameter.





